
The ECFS Patient Registry is a longitudinal study. It recruits all the prevalent confirmed cystic fibrosis
cases on 1st January 2008 and all the subsequent incident cases.
Only individuals who fulfil the diagnostic criteria below should be included in the registry as having a confirmed CF diagnosis:
OR
If the sweat test value is ≤ 60 mmolL−1, then at least 2 of these must be fulfilled:
This should be indicated in the data and communicated by email to: servicedesk@ecfregistry.eu.
and no further data should be included in the ECFSPR for the individual.
Reasons for reversal of diagnosis:
From January 2026, the ECFSPR will collect data about consenting people who have been designated CFSPID, including conversion to CF or a CFTR-RD* or to CF carrier / healthy. Data about CFTR-RDs or healthy carriers will not be collected, other than the conversion status.
OR
*CFTR-RD: Cystic Fibrosis Transmembrane Conductance Regulator Related Disorder
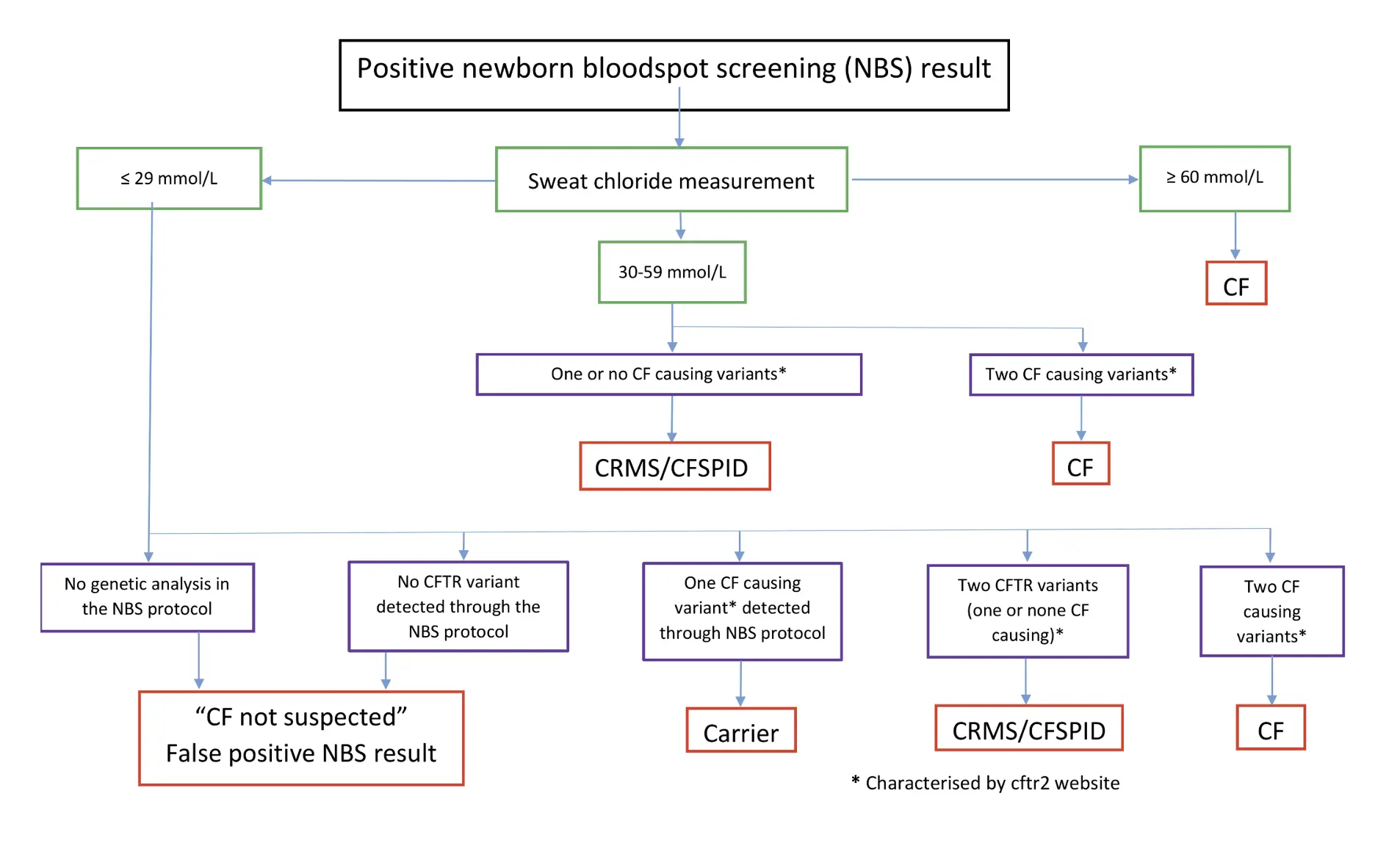
An algorithm for the designation of infants, following the positive newborn screening (NBS) result.
CF: Cystic fibrosis, CFTR: CF transmembrane conductance regulator, CRMS: CFTR-related metabolic syndrome, CFSPID: CF screen-positive, inconclusive diagnosis