
24 May 2025
3 min read
CFTR modulators are a groundbreaking class of medications that target the underlying cause of cystic fibrosis rather than just managing its symptoms. Until now, access to these modulators for very young children was limited due to a lack of safety and efficacy data in this age group. The EMA’s new recommendation is based on promising results from recent clinical trials, which showed improvements in lung function, nutritional status, and overall quality of life, along with a favorable safety profile.
CFTR modulators are a groundbreaking class of medications that target the underlying cause of cystic fibrosis rather than just managing its symptoms. Until now, access to these modulators for very young children was limited due to a lack of safety and efficacy data in this age group. The EMA’s new recommendation is based on promising results from recent clinical trials, which showed improvements in lung function, nutritional status, and overall quality of life, along with a favorable safety profile.
“This recommendation moves us closer to treating CF as a manageable condition from the earliest stages of life.”
Parents often feel overwhelmed after a CF diagnosis — particularly when it happens via newborn screening. The promise of early access to targeted treatment gives families more than hope; it gives them options. Instead of waiting for symptoms to appear, clinicians can now act proactively to improve long-term outcomes.
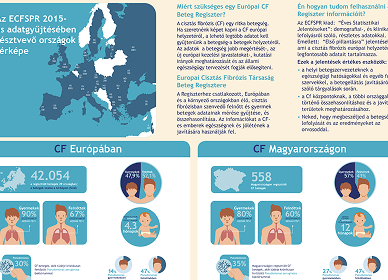
With standardized access, data harmonization becomes easier. Registries like the ECFS Patient Registry can begin to collect more robust, comparable data on very young patients receiving modulator therapy, helping to assess real-world impact across different settings and refine care models.

The CHMP’s recommendation will now be forwarded to the European Commission for formal approval. If granted, the new indication will become valid across all EU member states and EEA countries, further standardizing access to advanced CF care across the region.
With standardized access, data harmonization becomes easier. Registries like the ECFS Patient Registry can begin to collect more robust, comparable data on very young patients receiving modulator therapy, helping to assess real-world impact across different settings and refine care models.