
The ENRICH project is an ongoing multi-centre, longitudinal, observational study that will collect and analyse from 5,000 to 10,000 chest CT scans acquired during routine care; the data from these scans will enrich the ECFSPR with quantified markers of structural lung abnormalities.
The European Cystic Fibrosis Society Patient Registry (ECFSPR) annually collects clinical data from more than 55,000 people living with cystic fibrosis (PwCF) across Europe. It is a powerful resource for understanding the real-life impact of Cystic Fibrosis (CF) on the daily lives of PwCF and informing improvements in routine care. However the Registry, until now, has been unable to capture the direct, quantitative measures of structural lung abnormalities which are central to understanding the progression of lung disease.
Chest computed tomography (CT) scans, widely considered the “gold standard” for assessing structural lung damage, are already part of routine care in approximately half of CF centres in Europe. Chest CT have been shown to correlate strongly with important health outcomes such as lung function, frequency of exacerbations, quality of life and life expectancy. Despite their clinical importance, the interpretation of chest CT scans has been done visually, which does not give quantifiable data. With the use of automated CT scoring tools structural lung abnormalities can be objectively quantified. This allows for consistent assessment of disease severity, early detection of subtle changes, monitoring of treatment response and standardisation across timepoints and centres.
Through the ENRICH project, we can integrate these missing structural lung abnormality measures into the ECFSPR for the first time. This initiative bridges an important gap, enriching the registry with robust, quantitative imaging data that will strengthen both clinical research and patient care.
ENRICH will evaluate how structural lung abnormalities evolve in PwCF and how these changes relate to established markers of disease severity.
The project will compare the effects of different CFTR modulator therapies on the progression of structural lung disease, using quantitative imaging data from routine chest CT scans.
The study involves 18 CF centres from 12 countries in Europe that are part of the ECFS Clinical Trial Network (CTN). These centres routinely acquire chest CT scans as part of standard care and also submit patients' annual clinical data to the ECFSPR. The study includes PwCF who are already in the ECFSPR, are receiving one of the CFTR modulator therapies and have had repeated routine chest CT scans between 2017 and 2025. Below are the list of participating countries and their respective CF centres that contribute data and imaging to the ENRICH project:
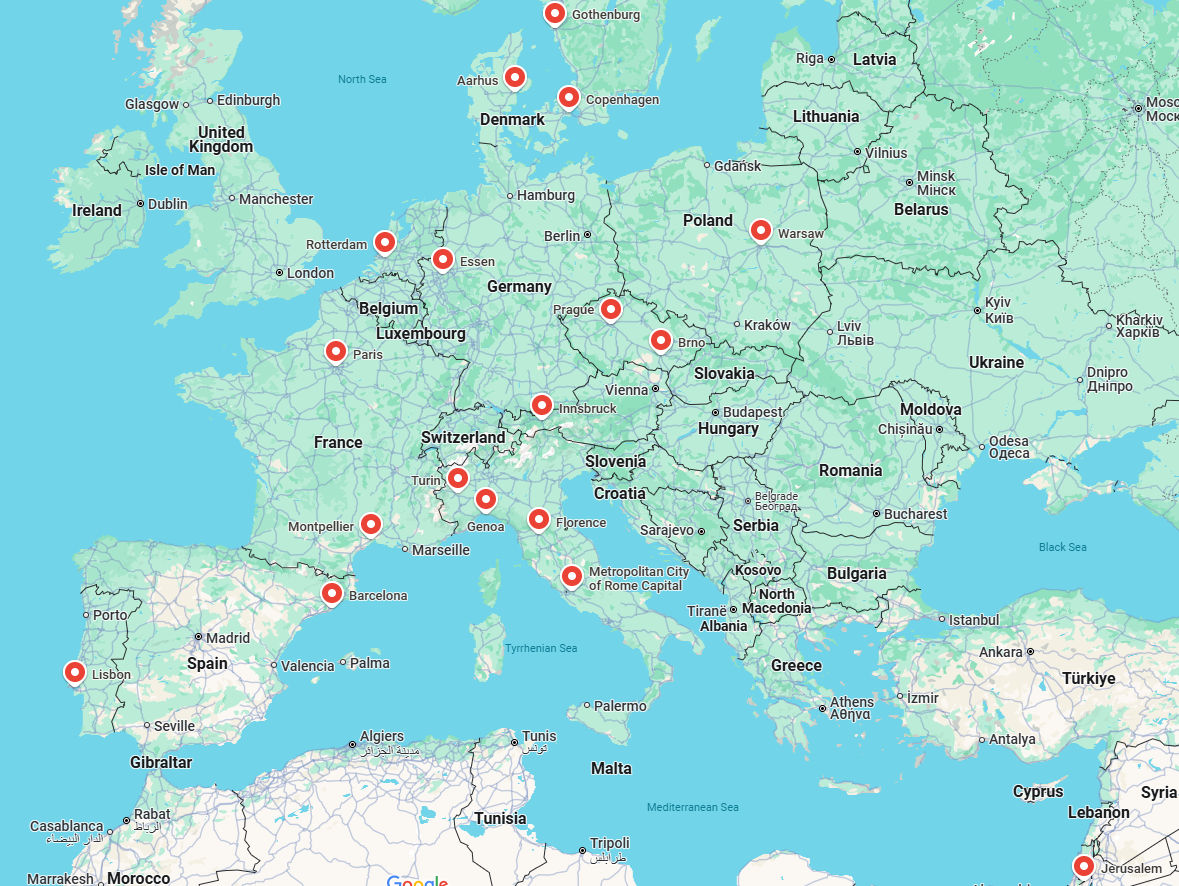
Figure 1. CF centers spread across Europe participating in ENRICH project
| Country | City | Institute Name |
| Austria | Innsbruck | Cystische Fibrose Zentrum, Medizinische Universität Innsbruck, Austria |
| Czech Republic | Brno | Clinic of children's infectious diseases, University Hospital Brno, Brno, Czech Republic |
| Prague | Cystic Fibrosis Centre, Motol University Hospital, Prague, Czech Republic | |
| Denmark | Aarhus | Department of Pediatrics and Adolescent Medicine, Aarhus Hospital, Aarhus, Denmark |
| Copenhagen | Department of Paediatrics and Adolescent Medicine,
Copenhagen University Hospital, Denmark |
|
| France | Montpellier | CHU de Montpellier - Hôpital Arnaud de Villeneuve, Montpellier, France |
| Paris | Cochin Hospital, Assistance Publique Hôpitaux de Paris, Paris, France | |
| Mucoviscidose et autres maladies épithéliales respiratoires par défaut de repliement protéique, Institut Necker Enfants Malades , Paris, France | ||
| Germany | Essen | Klinik für Pneumologie, Universitätsklinikum Essen (AöR), Essen, Germany |
| Israel | Jerusalem | Cystic Fibrosis center, Hadassah Medical Center, Jerusalem, Israel |
| Italy | Florence | Centro Regionale Toscano per la Fibrosi Cistica, Azienda Ospedaliero-Universitaria Meyer IRCCS, Florence, Italy |
| Genova | IRCCS Istituto Giannina Gaslini, Genova, Italy | |
| Rome | Pneumology & Cystic Fibrosis Unit, Bambino Gesù Children's Hospital, Rome, Italy | |
| Torino | Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino, Turin, Italy | |
| Netherlands | Rotterdam | Erasmus MC- Sophia, Rotterdam, Netherlands |
| Poland | Warsaw | Warsaw Cystic Fibrosis Centre, Dziekanow Lesny Paediatric Hospital, Warsaw, Poland |
| Portugal | Lisbon | CHULN- Hospital de Santa Maria, Lisbon, Portugal |
| Spain | Barcelona | Pediatric Pulmonology and Cystic Fibrosis center, Hospital Universitari Vall d’Hebron-VHIR, Barcelona, Spain |
| Sweden | Gothenburg | Drottning Silvias Barnsjukhus, Sahlgrenska Universitetssjukhuset, Gothenburg, Sweden |
The chest CT data are collected and analysed under the ENRICH project. Ethical approval and patient consent are obtained.
Inspiratory and Expiratory chest CT scans acquired in axial/transverse direction are accepted under if the reconstructions have overall slices ≥ 100, slice thickness ≤ 1.5 mm, without slice gap and no missing slices. Only pseudonymized scans are accepted.
The routine chest CT data of PwCF is collected from the participating CF centres of the ENRICH project through the Data Transfer Zone (DTZ) platform of Erasmus MC. This platform is a double-encrypted online application with no constraint on file size. The chest CT outcome file is formatted to be ECFSPR compatible and is shared back with the respective CF centres via the DTZ platform.
The images are analysed using the automated platform LungQTM and developed by Thirona B.V. in the Digital Research Suit platform of Erasmus MC. Below is a ist of the automated algorithms used and the chest CT outcomes included in the ENRICH project:
1. Bronchus-Artery analysis
a. Bronchus-artery ratios
b. Bronchus and artery dimensions
c. Bronchus generations
d. Bronchus-artery pairs
2. PRAGMA-AI
a. %Bronchiectasis
b. %Airway wall thickening
c. %Disease
3. Mucus plugging
a. Number of mucus plugs
b. Volume of mucus plugs
4. VERA
a. % normal
b. % emphysema
c. % airtrapping
d. % airtrapped emphysema
The European Cystic Fibrosis Society Patient Registry (ECFSPR) plays a vital role in the ENRICH project; we liaise with participating CF centres and national CF registries, address queries and support engagement throughout the study. In collaboration with OpenApp (the software company that created and manages the ECFSPR data collection platform), the ECFSPR has developed an add-on, dedicated Chest CT Module to facilitate the integration of the quantified chest CT outcome data. This platform is now fully operational and accessible to all ENRICH-participating CF centers and their respective national CF registries.
Erasmus – Lung Analysis
The LungAnalysis – Image Analysis Core Laboratory serves as the ECFS-CTN Computed Tomography (CT) Core Laboratory for the standardisation of chest CT image acquisition and centralised image analysis for clinical research and trials. Since 2012, LungAnalysis provides training services for spirometer-guided and technician-controlled chest CT scan procedures, ensuring standardised chest CT image acquisition. LungAnalysis also offers chest CT image analysis services for both investigator- and sponsor-initiated studies in clinical research and trials.

Principle investigator

PhD candidate
Cystic Fibrosis Foundation (CFF), USA

